Imaging of Bronchiectasis
Bronchiectasis is defined as irreversible dilatation of a portion of the bronchial tree. The three most important mechanisms that contribute to the pathogenesis of bronchiectasis are infection, airway obstruction and peribronchial fibrosis.
Imaging plays a pivotal role in the diagnosis of bronchiectasis. High-resolution computed tomography (HRCT) is the cornerstone in the radiological diagnosis of clinically suspicious cases. HRCT is the most sensitive and specific non-invasive method for diagnosing bronchiectasis. In addition to making the diagnosis, the pattern of disease on HRCT may enable one to limit the differential to a single/few specific causative entities.
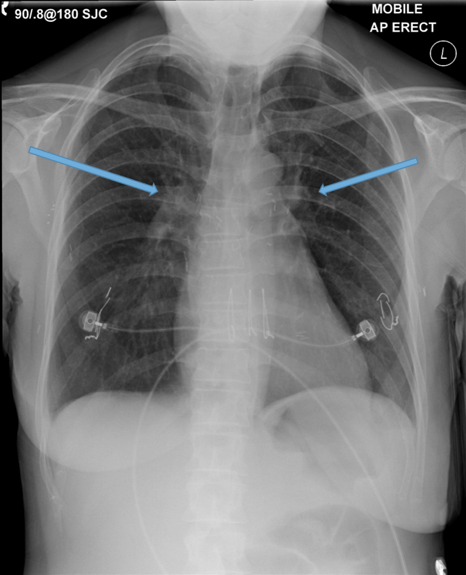
The CXR in affected individuals is often normal or shows non specific findings. Its role has largely been reduced to surveillance for intercurrent infection, progressive lobar collapse or suspected development of cavitary disease in patients with known bronchiectasis.
Certain descriptive terms have been used in reporting of bronchiectasis. These only describe the appearance of the involved airways but do not elude to a specific cause.
These terms include:
Cylindrical (tram track sign):
Signet-ring:
Varicose
Cystic or saccular
A cluster of thin walled cystic spaces
CXR findings
Direct signs
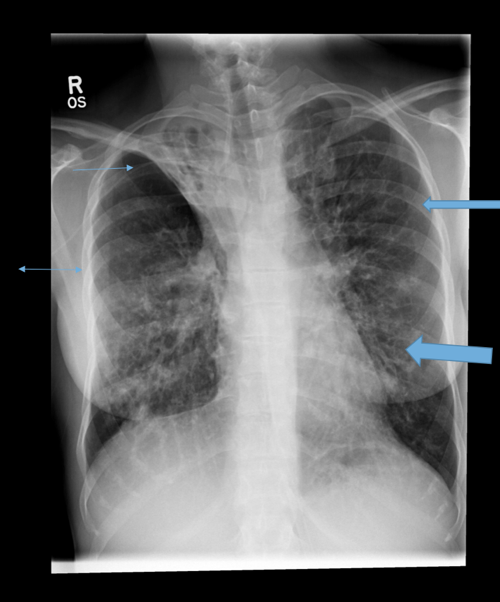
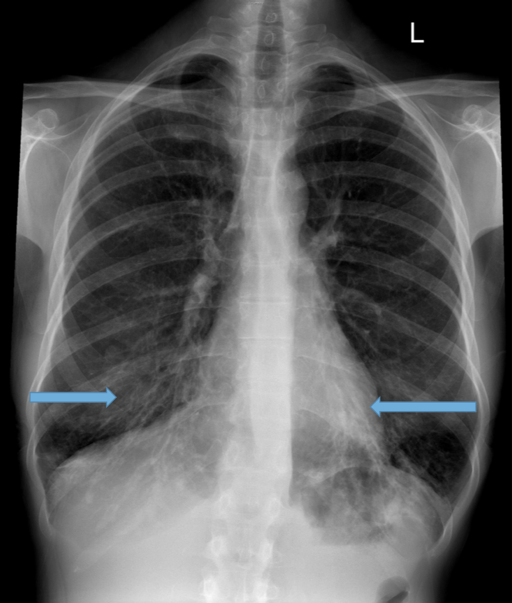
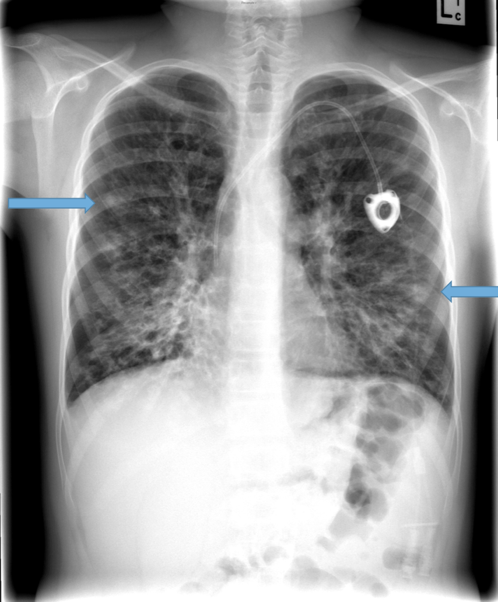
Parallel line opacities (tram tracking) Fig 1a, b
Tubular opacities (mucous filled bronchi) Fig 2
Ring opacities (dilated end-on bronchi) Fig 1
Indirect signs
Lobar atelectasis (secondary to mucous plugging) Fig 1
Compensatory overinflation of the less affected lobe/lung Fig 1
Multidetector CT Imaging Technique
Multidetector CT scanners enable volumetric data acquisition with scans obtained through the entire thorax in a single breath-hold. This can be obtained in a few seconds or less thereby minimising respiratory and cardiac motion artefact. Images can then be reconstructed in multiple planes and slice thicknesses. It is recommended that thin slice images be reconstructed between 1-2 mm thick every 10mm, i.e. non contiguous scans. With this technique there are gaps in the viewed images therefore a second series should also be provided from the volumetric dataset containing thicker (5mm) but contiguous slices. This ensures that additional pathologies such as small lung nodules or neoplasms are not inadvertently missed.
Occasionally it may be helpful to also acquire expiratory images to assess the degree of air trapping, both focal and diffuse. Although this does create issues around increased radiation dose, in certain clinical situations it can be justified, for example the clinician may be suspicious for small airways involvement which is not readily appreciated on the inspiratory HRCT. Expiration can exaggerate these findings by demonstrating mosaic/geographic regions of air trapping. A specific condition where this may be seen is obliterative bronchiolitis.
HRCT interpretation:
Bronchial dilatation.
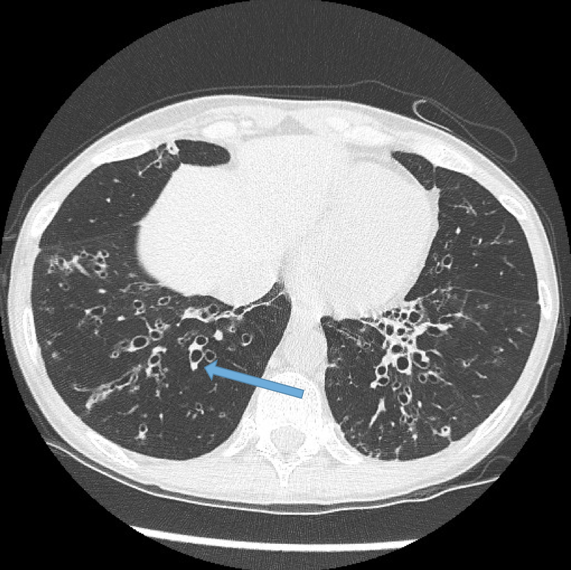
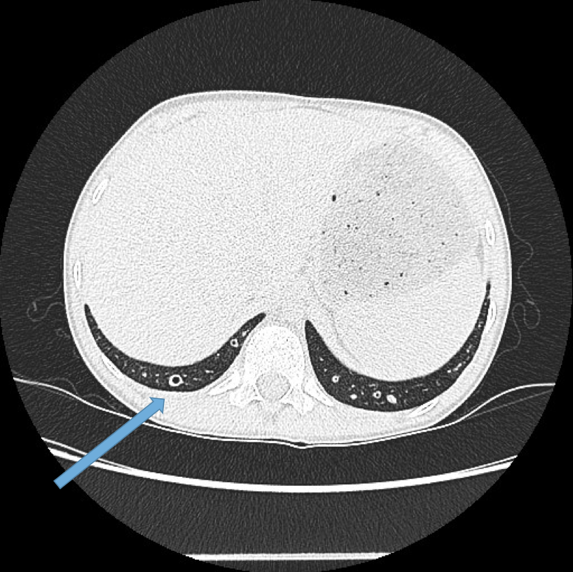
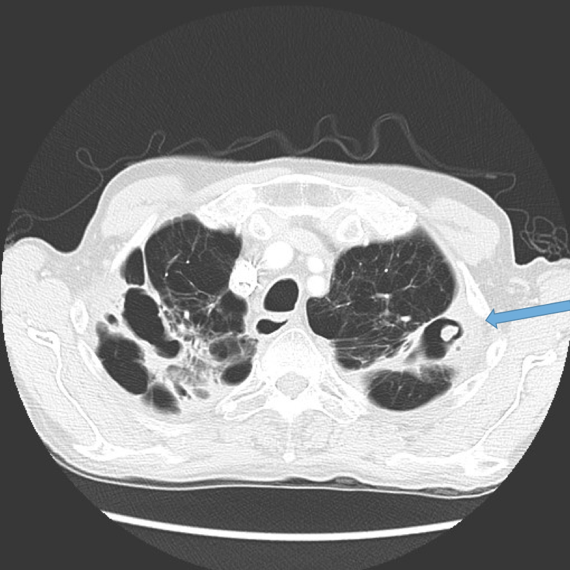
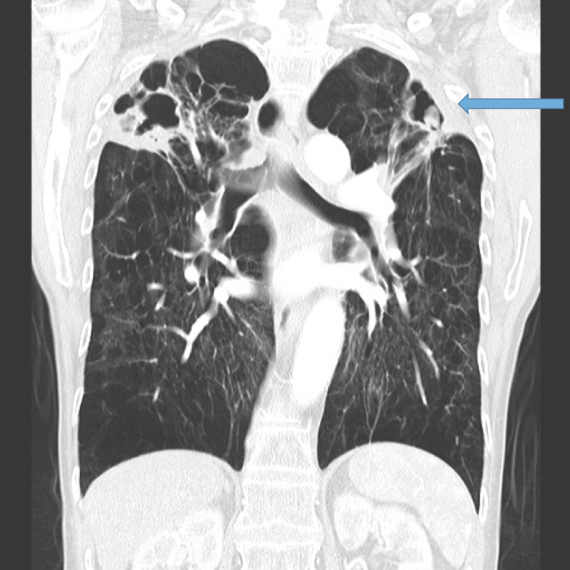
This is the most important finding to establish a diagnosis of bronchiectasis. Dilatation is defined as the internal bronchial diameter greater than that of the adjacent pulmonary artery. This is referred to as the signet-ring sign. Fig 3a,b, c, d
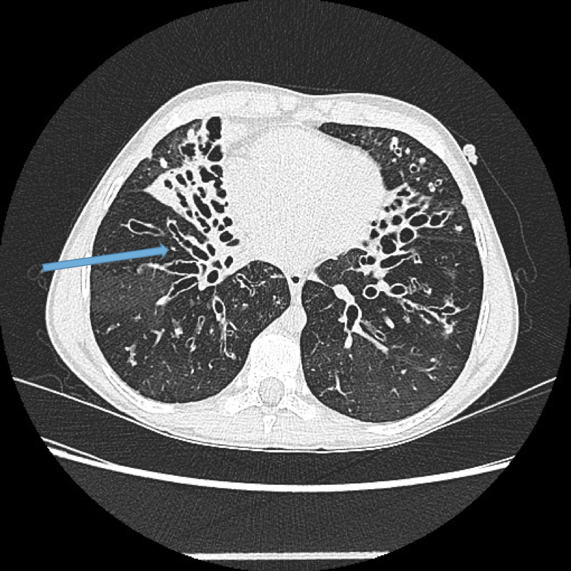
Lack of bronchial tapering.
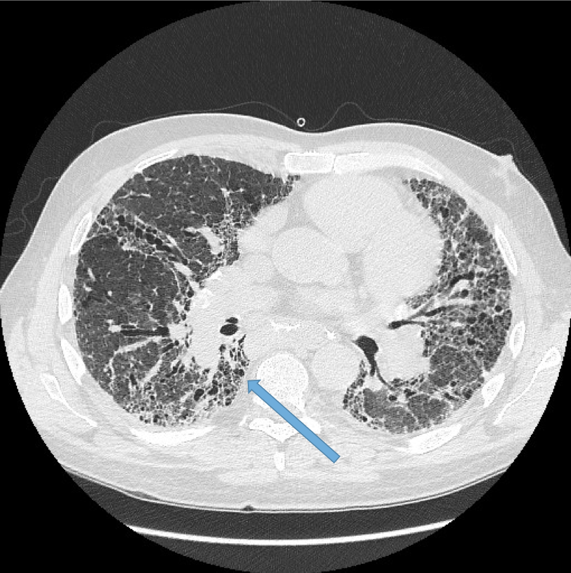
Normal airways diminish in calibre as they extend toward the lung periphery. Fig 4
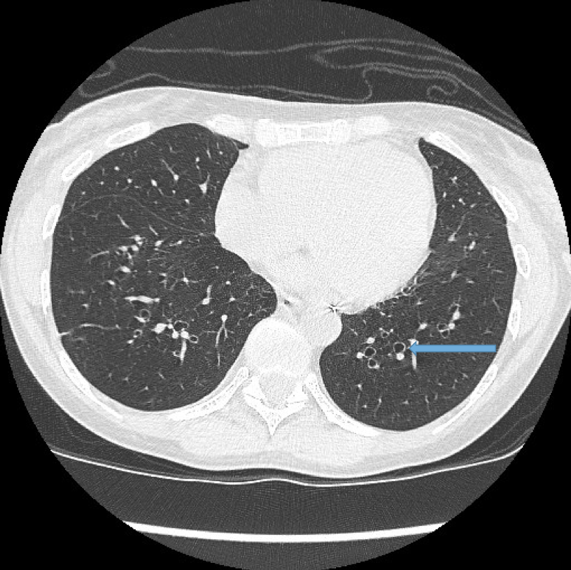
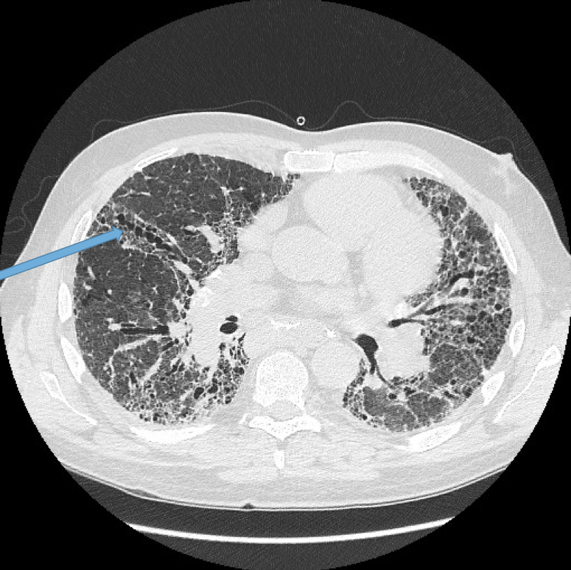
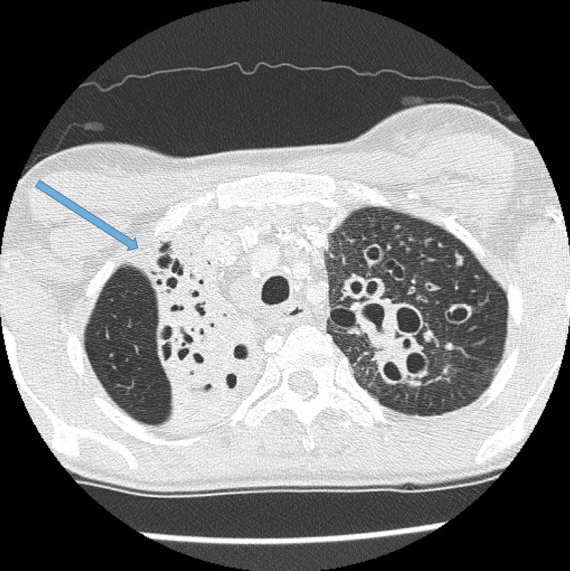
Visualisation of bronchi within 1 cm of the costal pleura.
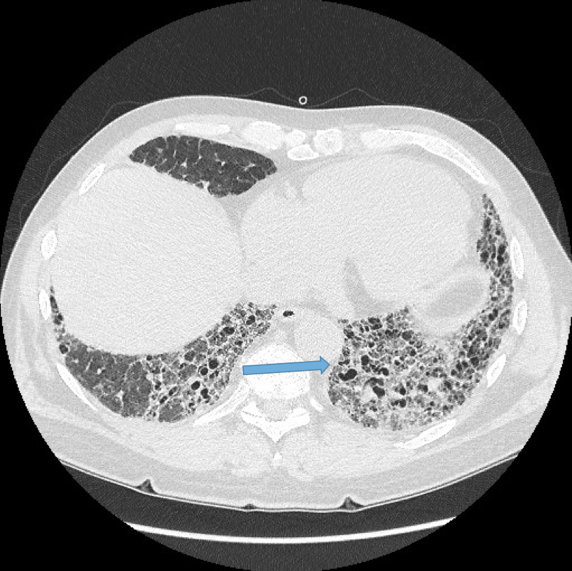
Normal airways should not be visualised this far out in the lung periphery. Fig 5
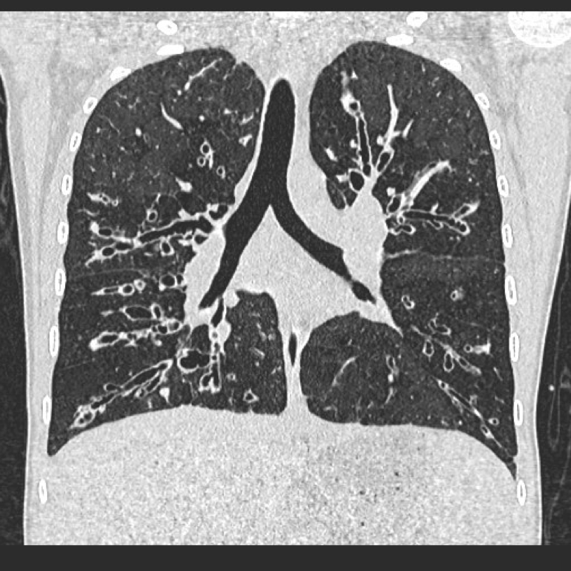
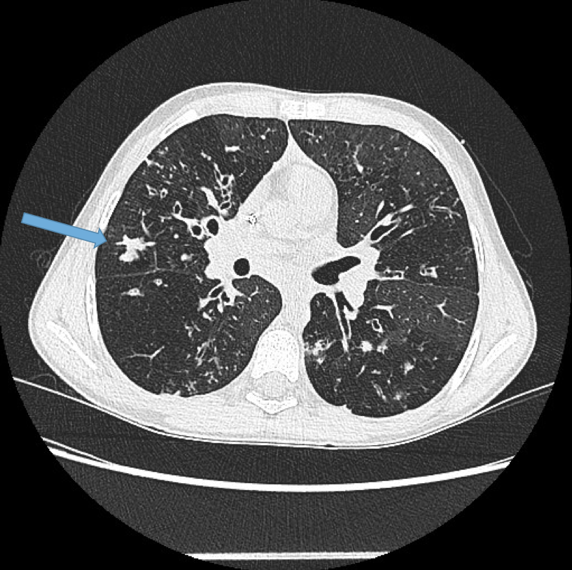
Visualisation of bronchi abutting the mediastinal pleura. Fig 6a, b
Mucoid impaction. May be seen as lobular, tubular branching structures. The mucous may become of high density due to chronic inspissation. Fig 7
Bronchial wall thickening. This is non specific and may be reversible if there is an intercurrent infection giving rise to acute bronchitis. Fig 8
Mosaic lung attenuation. This term is used to describe heterogeneous lung density due to air trapping in the affected lung segments and as a result has a geographic distribution. This finding can be produced or exaggerated on expiration. Fig 9.
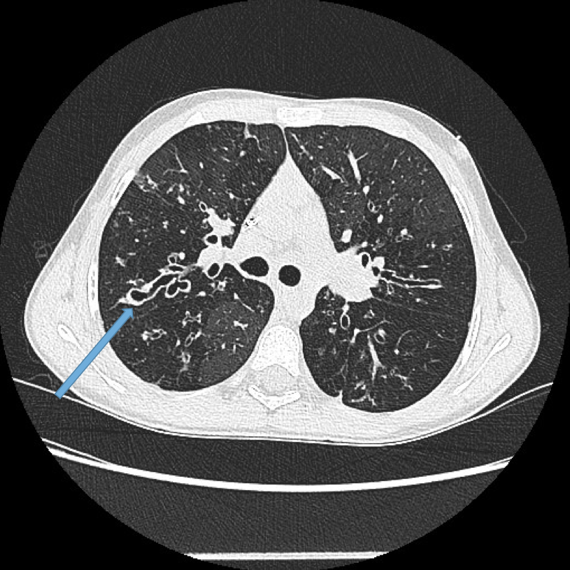
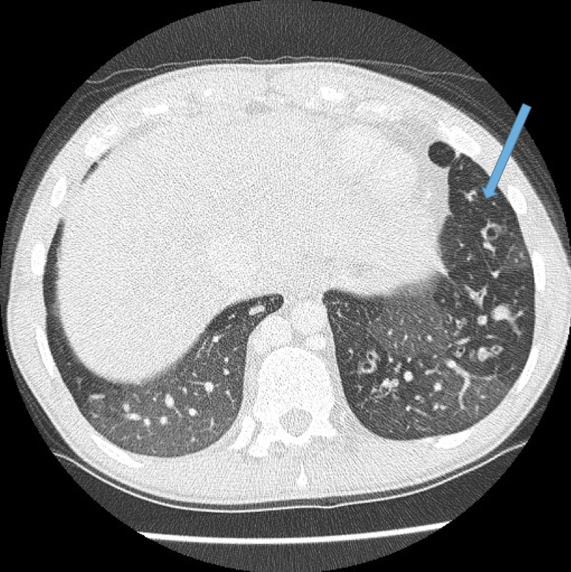
Vascular abnormalities.
Dilated bronchial arteries. These are best demonstrated post administration of intravenous contrast. These tortuous vessels extend along the central airways toward the hila. It is these vessels that are often responsible for haemoptysis, a symptom these patients may describe. Fig 10
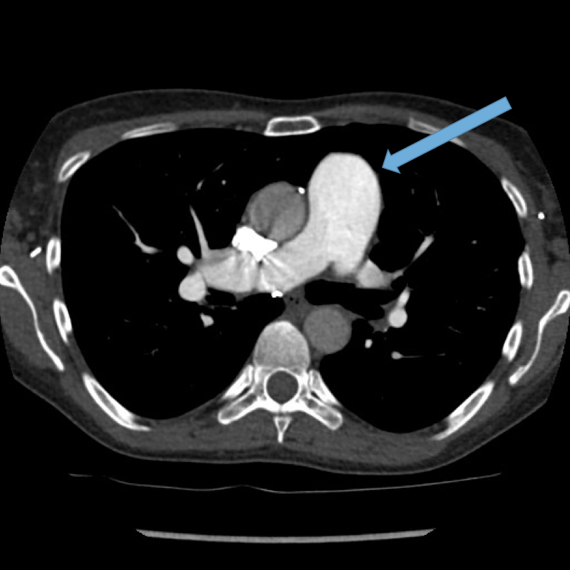
Dilated main pulmonary artery. This ususally indicates underlying pulmonary hypertension as a sequelae of chronic, severe lung disease. Fig 11, 12
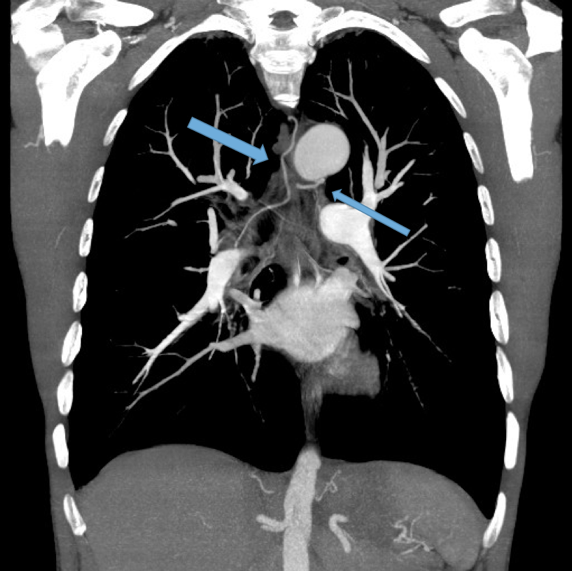
Other findings
Lobar collapse Fig 13a, b
Mycetoma formation (Fungus ball) Fig 14a, b, c
Aspergillus fumigatus is a fungus that may colonise dilated airways or bullae/cavities. It is an important cause of haemoptysis.
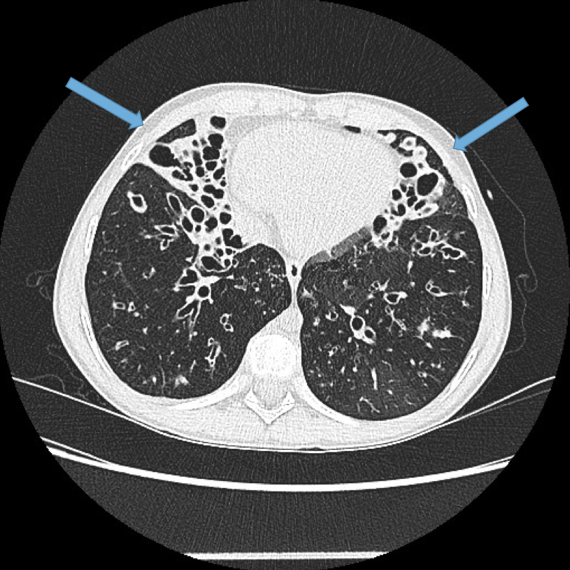
CT features predicting mortality (Loebinger et al 2011):
increased wall thickness
emphysema
dilation severity
small and large airway plugging
mosaicism
Conclusion
HRCT continues to be the gold standard for establishing the diagnosis, the anatomic extent and severity of bronchiectasis.
CXR is utilised in the follow up of these patients once a diagnosis has been established, particularly when they present acutely with an exacerbation. As bronchiectasis is a chronic illness often requiring multiple hospital admissions throughout life for acute exacerbations, we must remain cognisant of the cumulative radiation dose to each patient. In difficult to interpret CXR’s or patients who fail to improve post conventional antibiotics, HRCT can be utilised to assist in ongoing management by ruling out unusual infections such as mycetoma, demonstrate new or progressive lobar collapse or to guide bronchoscopy.








