Medications for Bronchiectasis
Antibiotics
Antibiotics are central to the management of bronchiectasis. The selection of the initial antibiotic approach should be driven by symptoms, symptom escalation, the presence of mucopurulent sputum and the availability of lower airway culture results from sputum (or where available or occasionally necessary, bronchoscopic sampling). Selection should be guided by previous antibiotic responses, allergy, drug tolerability, antibiotic susceptibility patterns and clinical severity.
Antibiotics (oral, intravenous or nebulised) can be used in three situations:
To attempt eradication of new airway isolates
To treat exacerbations
As a long term maintenance for suppression of chronic colinisation
The use of inhaled antibiotics is challenged by a poor evidence base. The addition of inhaled tobramycin to ciprofloxacin for the treatment of acute exacerbations of Pseudomonas Aeruginosa colonised bronchiectasis as an example revealed a superior microbiological response but no clinical superiority at 21 days when compared with ciprofloxacin and placebo and so further patient numbers may be required to confirm benefit (Bilton 2006).
A medium sized randomised trial of nebulised Colistin for acute exacerbations in Pseudomonas Aeruginosa colonised bronchiectasis has also failed to achieve the primary outcome of time to first exacerbation, although encouragingly there was benefit in those in whom a high level of treatment adherence was observed (Haworth 2014).
Through these trials we see a diversity of responses and outcomes that preclude population based treatment recommendations. There are however opportunities for interventions for the individual which should be carefully considered on a case by case basis with close monitoring of clinical effect. The selection of treatments will be based on clinical phenotype based on features including lung function, bronchodilator responsiveness, symptoms, exacerbation frequency and microbial colonisation.
The challenges in managing Pseudomonas Aeruginosa are described in the following review article (Wilson et al 2016) Challenges in managing Pseudomonas Aeruginosa
Eradication of new isolates. The isolation of haemophilus, Strep. pneumoniae, Staph aureus (not MRSA) and in some cases, new isolates of Pseudomonas aeruginosa should prompt an appropriate trial of antibiotics with eradicative intent.
The optimal eradication regime for Pseudomonas aeruginosa has not been determined however, in practice, two weeks of oral ciprofloxacin is often used. This may be escalated in cases of persistently positive cultures. Specialist advice is recommended.
There is currently no evidence to support the eradication of other organisms.
Maintenance suppression of persisting microbial colonists. Once established in the airway long term colonists may be difficult to eradicate. A therapeutic trial of pathogen-targeted inhaled antibiotics (Tobramycin / Colistin/ Gentamicin / Ciproflxacin ) may be considered in selected patients e.g. those with established Pseudomonas aeruginosa colonisation and frequent exacerbation. However, the use of inhaled antibiotics is challenged by limited but evolving evidence. Also, nebulised antibiotics are associated with a 10% – 30% risk of bronchospasm and therefore specialist management is recommended. Bronchodilators may be required prior to nebulised antibiotics.
See Correct use of Medications for the administration of antibiotics via a nebuliser.
Maintenance suppression of recurrent exacerbations.
Long-term oral antibiotics can also be considered for patients with recurrent exacerbations who are otherwise optimally managed, but should not be prescribed routinely (ERS Guidelines 2017).
Macrolides exert immunomodulatory and antibiotic effects, and have been shown to reduce exacerbation frequency.
Macrolide antibiotics for bronchiectasis
Airway infection and inflammation are key elements in the development and progression of disease in patients with bronchiectasis. The current concept of pulmonary exacerbations is that they arise from alterations of the airway microbial ecosystem (dysbiosis) leading to an abnormal host immune response, excessive airway inflammation and disordered microbial environment (Dickson et al 2014). Macrolide antibiotics target both inflammation and infection and have been shown to have beneficial clinical effects in patients with bronchiectasis.
Macrolide antibiotics (erythromycin, clarithromycin, roxithromycin, azithromycin) have many antimicrobial, anti-inflammatory and immunomodulatory properties (Kanoh and Rubin 2010). They are also efficiently delivered to sites of infection and achieve high tissue concentrations, particularly for azithromycin (Parnham et al 2014). This unique combination of characteristics is thought to explain the effectiveness of macrolides in bronchiectasis.
Clinical Trials
Three major randomised controlled trials in adults and one in children have shown that azithromycin and erythromycin are effective in preventing pulmonary exacerbations (reduced by 40-60%) in patients with bronchiectasis (Wong et al 2012, Altenburg et al 2013, Serisier et al 2013, Valery et al 2013). Meta-analyses of these and smaller studies also show modest improvements in quality of life and lung function (Wu et al 2014, Gao et al 2014).
Adverse effects
Gastrointestinal effects (mainly diarrhoea) are common with azithromycin but are generally mild.
Hearing impairment has not been evaluated in bronchiectasis but has been reported in a study of azithromycin in COPD patients (Albert et al 2011). Hearing decrement was increased by 5% in the azithromycin group as compared to the placebo group.
Macrolides have the potential to cause cardiac arrhythmias but the risk is very small with oral treatment and greatest with intravenous treatment. Caution should be taken with patients who have prolonged QTc interval.
Resistance to macrolides is very likely to develop with prolonged macrolide treatment. However, overall macrolide treatment is beneficial for patients with bronchiectasis and the negative consequences of macrolide resistance for individual patients treated with macrolides are unclear.
Who should be treated?
This should be assessed on a case by case basis and the benefit to risk ratio for the patient needs to be considered carefully. In addition, the risk to the community of increasing macrolide resistance in pathogenic bacteria such as Streptococcus pneumoniae, Staphylococcus aureus, Haemophilus influenzae, and nontuberculous mycobacteria should be considered.
Patients with frequent exacerbations (3 or more exacerbations in the past year) and poor quality of life may be considered for macrolide therapy (Hill 2016).
Treatment doses
Dose regimens vary according to different studies and have not been standardised.
Azithromycin
500 mg 3 times a week (Monday, Wednesday, Friday)
250 mg 3 times a week (if patient is unable to tolerate higher dose)
250 mg daily
30 mg/kg once a week in children
Erythromycin
Erythromycin ethyl succinate – 400 mg twice daily
Erythromycin stearate – 150 mg twice daily
Duration of treatment
The optimal duration of treatment is not clear. Positive clinical trials have treated for 6 or 12 months. The maximum benefit of macrolide treatment is thought be attained after at least 3 months of treatment. Therefore treatment durations of between 3 and 12 month could be considered and some patients may require longer term treatment.
A pragmatic approach to treatment is to give macrolide treatment over the cooler months, when the risk of exacerbations is highest, with a drug holiday over the summer months.
Checklist prior to starting treatment
Frequent exacerbations (3 or more exacerbations in past year)?
Exclude non-tuberculous mycobacterial infection (sputum culture x3)
Assess cardiac risks (QTc interval, arrhythmia) – ECG
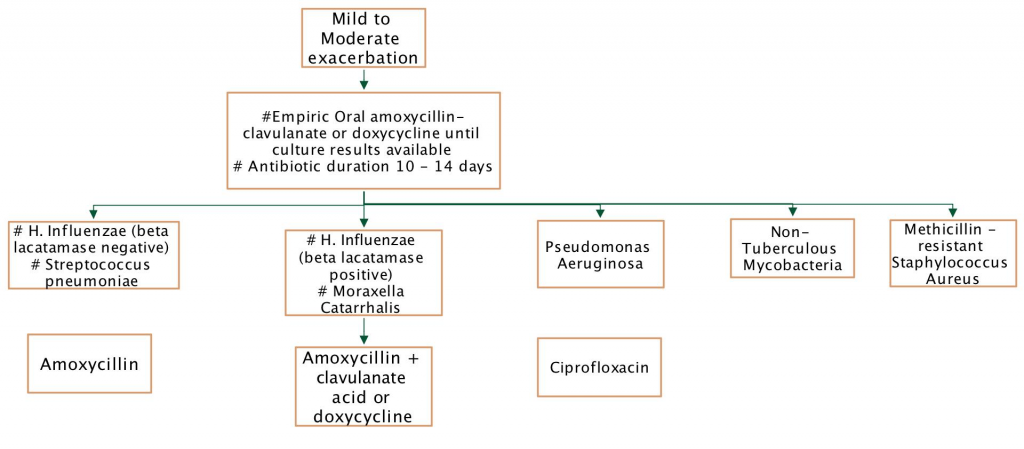
Antibiotic use – mild to moderate exacerbation
Antibiotic use – severe exacerbation

Antibiotic strategies in bronchiectasis
| Inhaled | Oral | Intravenous | |
|---|---|---|---|
| Eradication | Yes | Yes | Yes |
| Suppression | Yes | Maybe | No |
| Maintenance | Yes | Maybe | No |
| Anti-inflammatory | Maybe | Yes | No |
| Mild exacerbation | Yes | Yes | No |
| Severe exacerbation | Yes | Yes |
Outpatient management of exacerbation. Oral antibiotics are prescribed for 10-14 days based on available airway microbiology results. Close follow-up to assess treatment response is necessary.
Inpatient management of exacerbation. Failure to respond to oral antibiotics, severe exacerbation or occasionally for relentless slow increase in symptoms or fall in lung function, may prompt admission (in-patient or hospital in the home) for intensified IV antibiotic therapy.
Mucoactive agents
isotonic saline (0.9%)
hypertonic saline (3% – 7%)
Mannitol
These agents, which increase hydration of the airway surface, alter mucus rheology and increase mucociliary clearance are not currently routinely recommended for people with bronchiectasis due to the lack of research evidence.
Clinically, significant benefits can be achieved so the following patient scenarios may benefit from a therapeutic trial:
frequent exacerbations
difficulty clearing secretions
chronic colinisation, in particular Pseudomonas aeruginosa
substantial sputum burden
Recombinant human deoxyribonuclease, used frequently in people with cystic fibrosis (CF), is contraindicated in non-CF bronchiectasis.
While bronchiectasis in CF and non-CF patients shares some similarities there are also significant differences. Some of these differences appear counterintuitive and so the simple grandfathering of treatments from the CF evidence base to non-CF bronchiectasis is inappropriate and may be potentially harmful.
Dornase, the recombinant DNase as an example has been evaluated in two trials showing no benefit in one trial and a worsening in FEV1 and increase in exacerbation frequency in the other in Dornase treated subjects (O’Donnell 1998, Wills 1996). In contrast, case reports however have suggested some benefit for Dornase treatment in primary ciliary dyskinesia (Desai 1995, El-Abiad 2007).
Bronchodilators
Inhaled bronchodilators should not be prescribed routinely but used only on an individual basis if significant reversibility has been identified. In vitro studies suggest salbutamol may have a positive impact on mucociliary function and ongoing use will be guided by patient benefit.
Short acting bronchodilators may be prescribed prior to the inhalation of mucoactive agents and/or inhaled antibiotics if the patient demonstrates bronchoconstriction induced by the mucoactive agent.
Inhaled corticosteroids
Inhaled and oral corticosteroids should not be prescribed routinely unless there is an established diagnosis of coexisting asthma or COPD. Indeed steroids may have a negative impact on local immune responses and frequently the challenge lies in trying to wean inhaled steroids from patients on steroid therapy prior to confirmation of a diagnosis of bronchiectasis.








