Pathophysiology
As bronchiectasis is an acquired disorder, its pathophysiology is commonly described as distinct phases of infection and chronic inflammation. The interaction between these phases establishes a vicious circle (Fig. 1) in which the end result is the destruction of the bronchi and the accompanying clinical symptoms.
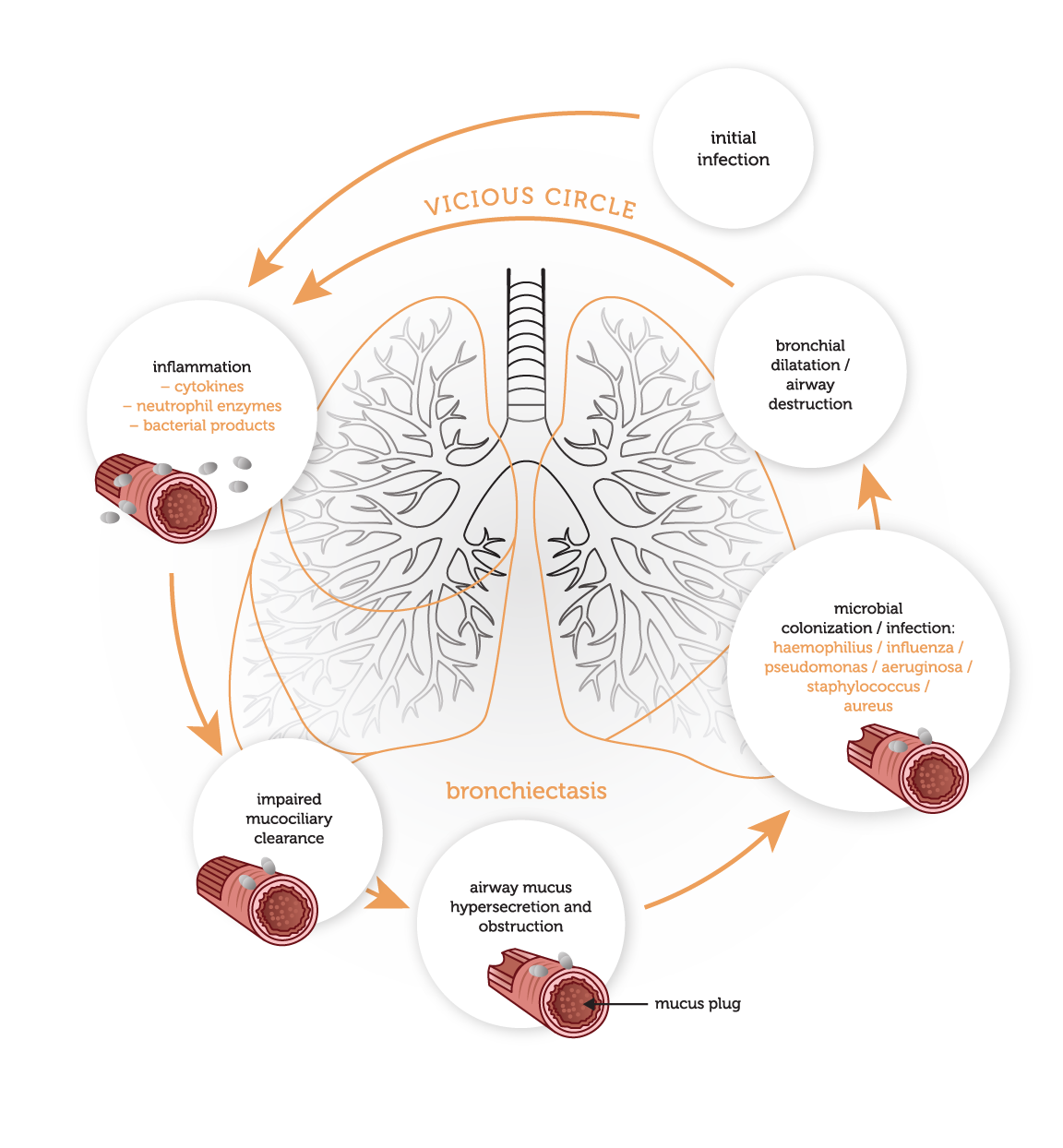
The first stage in the development of bronchiectasis is an initial infective insult to the airways, which triggers a mucociliary response. Micro-organisms trigger the release of toxins and an inflammatory response within the airways. This inflammatory response includes the release of neutrophils, lymphocytes and macrophages within the bronchial lumen. Neutrophils also alter the function of the cilial epithelium, leading to changes in cilial beat frequency and mucous gland hypersecretion. Both processes compromise mucociliary clearance.
This loss of mucociliary transport renders the airways susceptible to microbial colonization. Neutrophils are also believed promote this process by allowing bacterial adherence to the lung epithelium. In response to this colonisation, a cycle of an intense chronic inflammatory response is triggered. This encourages further release of inflammatory mediators, which facilitate neutrophil migration to the bronchial lumen and mucosa. Neutrophils play a central role in the tissue damage in bronchiectasis, by releasing mediators (including inflammatory cytokines, elastases and matrix metalloproteinases) which destroy the bronchial elastin and other supporting lung structure, leading to permanent dilatation of the bronchi (Fig.1). Airway walls become thickened with normal mucosal and muscular layers substituted by oedema, ulceration or fibrosis. In proximal airways, the structural cartilage may be diminished, provoking a reduction in supportive structure. These changes in airway structure further contribute to pooling of mucus, and the self-perpetuating cycle of infection and inflammation, which promotes progressive airway damage and recurrent infections (Fig. 2). Depending on the cause of bronchiectasis, the extent of the disease may be localised and focal or diffuse.

Although the majority of individuals with bronchiectasis have impaired mucociliary clearance and excess sputum production, not all patients are persistently colonised with bacteria. Bacterial colonisation and markers of inflammation in sputum are intermittent in most people. Patients often remain colonised with the same micro-organism during acute exacerbations as well as a stable clinical state.
Common microbials which may cause infections in bronchiectasis are:
Gram-negative:
Pseudomonas aeruginosa
Haemophilus influenza
Moraxella catarrhalis
Klebsiella pneumonia
Achromobacter
Stenotrophomonas maltophilia
Alcaligenes
Serratia marcescens
Escherichia coli
Steptococcus pneumoniae
Staphylococcus aureus
Mycobacterium avium complex
Mycobacterium abscessus
Aspergillus
Candida
Coronavirus
Rhinovirus
Influenzae A and B
The severity of airway obstruction and rate of decline in lung function may be influenced by the colonizing microorganisms. Individuals who show signs of colonization with Pseudomonas aeruginosa have had more extensive disease on HRCT, greater airflow obstruction and a faster rate of decline in airflow obstruction. Haemophilus influenzae is also known to induce detrimental effects on lung function.








